Neurons on Acid
How psychedelics can alter neuronal structure to restore lost synapses
This post is also available in:
 German
German
 Français
Français
 Italiano
Italiano
 Português
Português
 Ελληνικα
Ελληνικα


A new class of drugs, the psychoplastogens, may herald a new era in depression treatment.
Depression is a staple of modern times, with approximately 350 million people suffering from the disease worldwide. It is the most common mental health condition, causing a range of symptoms including prolonged sadness, apathy and anhedonia, and feelings of guilt or low self-worth. Severe depression can even cause suicide, and it increases people’s risk of death from other causes too. According to the World Health Organization, depression will rank second in global disease burden in 20201. In fighting the high societal costs associated with it, medical research on depression is currently facing two key challenges: We still do not fully understand the neurobiological mechanisms underlying depression, and many of the available treatments are either ineffective or only partially effective2.
Selective serotonin reuptake inhibitors (SSRIs) are the most prescribed class of antidepressant. However, only 56% to 60% of patients respond to this type of therapy at all3. Moreover, these drugs only begin to work after about two to four weeks, and many patients report side effects like dry mouth, weight gain, headaches, and loss of sexual desire4. The lack of effective therapies for depression is also due to the fact that even after decades of research, there is still no consensus on what causes it and which neurobiological mechanisms are affected.
Still, one important insight from basic research has been that exposure to chronic stress is associated with developing depression. This has driven more research for effective therapies5,6. While recognizing of the difficulties in generalizing pre-clinical findings to humans, animal and laboratory experiments can illuminate how stress impacts the brain and individual neurons. Such research has shown that exposure to stress can profoundly affect structural plasticity, which is the capacity of neurons to alter their shape when stimulated. In most neurons, stress exposure reduces the number of dendritic spines, the main places where neurons connect and communicate (see Figure 1).
The problem increases in complexity with the observation that stress impacts the brain in a region-specific manner7,8. In the amygdala, an important center for emotional processing, stress leads to increased complexity of neuronal morphology, due to an increase in the number of synapses9. But in other brain regions like the hippocampus and the pre-frontal cortex— important centers for decision-making and processing declarative memories —, stress reduces complexity. Synaptic contacts decrease, a phenomenon known as “synaptic stripping”6.
Cornered by the limited success of currently available treatments for depression, medical and scientific communities are now reaching out to psychedelic compounds, hoping they herald a new era in the treatment of psychiatric diseases. In a pioneering clinical trial, psilocybin has been shown to alleviate symptoms of depression for at least two months11. Remarkably, these studies involved patients with treatment-resistant depression. Moreover, ketamine, improves symptoms almost immediately and received approval from the US Food and Drug Administration in 2018, designating it as a “breakthrough therapy” for depression.
Recall that stress induces changes in neural morphology. Could psychedelic compounds have the capacity to reverse such changes, and might this be linked with their therapeutic effects? Recently, a study from the lab of David Olson at the University of California, Davis, tackled this question by investigating the effect of psychedelic compounds on neuronal morphology. They investigated changes in dendritic length and branching, as well as spine number and morphology. Like tree branches, dendrites are cellular “branches” that increase the reach of a given neuron and, thereby, its connectivity (see Figure 1). According to Olson’s work, psychedelic compounds from distinct classes have the property to increase the number of synaptic contacts, inaugurating a new substance classification: the psychoplastogens12.
Psychoplastogens
The term “psychoplastogen” refers to compounds that have the capacity to alter the morphology of neurons, also referred to as enhancing neuronal plasticity. In the study from David Olson’s lab12, the authors asked whether psychedelic compounds from different classes (and therefore with distinct pharmacological targets) can alter the morphology of neurons, therefore altering the probability of contact between neurons. The group used in-vitro methods, meaning they took living neuronal cell cultures from rat brains and incubated them with different concentrations of psychedelic compounds. They showed that LSD-25, MDMA, DMT, ketamine, psilocin (the active form of psilocybin), and other psychedelic compounds could induce changes in the number of synaptic contacts, as well as in the structure of neurons, by increasing the number and length of their dendritic branches. Moreover, based on the results obtained from the in-vitro experiments, the authors tested whether DMT could also induce alterations of neuronal morphology when injected into a living animal. Similar to the in-vitro data, they found that injected DMT also promoted an increase in the number of spines in the rat prefrontal cortex. This effect was accompanied by changes in neuronal activity, demonstrated by electrophysiological recordings of brain slices from rats that received DMT.
What is so surprising about this study is that all the tested psychedelic compounds displayed similar psychoplastogenic effects, despite targeting different classes of receptors. Naturally, the research group went on to investigate the underlying mechanisms promoting these psychoplastogenic effects. It is well known that certain messenger molecules, so-called ‘brain factors’, can induce changes in neuronal morphology. Of these, brain-derived neurotrophic factor (BDNF) is one of the most studied. BDNF is a member of the neurotrophin family of growth factors, playing a key role in neuronal survival, growth, and the differentiation of new neurons and synapses. It is abundant in brain regions involved in learning and higher cognition, such as the hippocampus and different cortical areas13. Interestingly, exercise can induce BDNF production and, conversely, stress can disrupt it14,15. In the present study, the authors found that inhibiting BDNF function through blocking the TrkB receptors to which it binds completely abolished the psychoplastogenic effects of compounds like LSD, DMT, and MDMA. This suggests that BDNF signaling might be a common mechanism underlying the effects of different psychedelic compounds on structural plasticity.
These results are compelling, but one question remained: would the same psychoplastogenic effect occur when psychedelic compounds are given to animals subjected to stress? Recently, a further study has shed light on this question16. The authors used a chronic stress exposure protocol, which is known to induce depressive-like behavior accompanied by a reduction of dendritic spines in neurons of the prefrontal cortex. Employing two-photon imaging, they tracked the fate of a subset of dendritic spines in the prefrontal cortex and showed that some of them disappeared after chronic stress. Interestingly, a single antidepressant dose of ketamine was able to rescue the spines that were lost due to chronic stress. Moreover, the authors went on to show that the ketamine-induced restoration of lost spines is, in fact, crucial for the behavioral effects of the drug. Overall, ketamine treatment restores lost spines and normalizes microcircuit activity in the prefrontal cortex, leading to the remission of depression-like behavior in mice. Figure 1 provides a graphical representation of the main findings from both studies12,16.
The results discussed above warrant further questions: Is the effect of ketamine region-specific? If yes, does ketamine also enhance structural plasticity in brain regions like the hippocampus and the amygdala? And given that other psychedelic compounds, such as psilocybin and MDMA, also quickly improve depression, can they also rescue dendritic spines that were lost due to chronic stress?
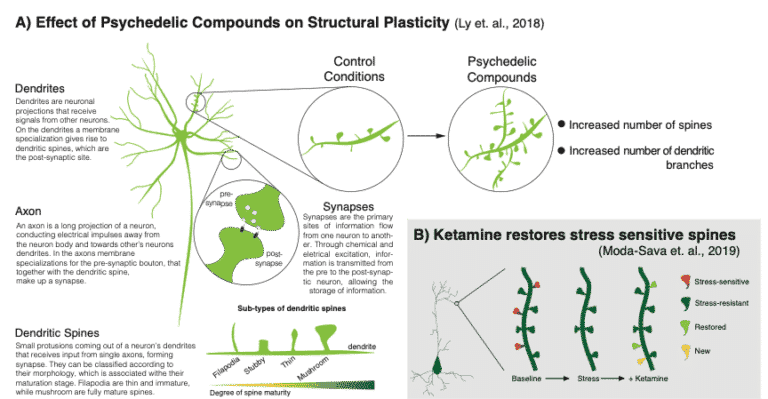
Figure 1: A brief explanation of relevant cellular neuroanatomical structures. Graphical representation of the major findings from A) Ly and coworkers12 and B) Moda-Sava and coworkers16 – adapted from Beyeler17.
Could structural plasticity accompany the psychedelic experience?
The studies discussed above suggest another question: Could structural plasticity accompany the psychedelic experience? To date, neuroimaging methods employed in human brain research do not confer enough resolution for visualizing dendritic spines. Nevertheless, several studies have looked at the effect of psychedelic substances on the brain using a variety of neuroimaging methods. From the seminal work of Carhart-Harris and coworkers in 201618, we have learned that LSD alters brain blood flow, electrical activity, and network communication and that this correlates with its subjective effects. This raises the question of whether structural plasticity could occur in a timeframe that correlates with the psychedelic experience. A related question is whether it is responsible for the long-term effects of such an experience, sometimes called the “afterglow effect.”
Basic research on the psychoplastogenic effects of psychedelic drugs contributes greatly to our understanding of their effects on the brain. Ly et al.12 published the first study to systematically show the effects of different chemical classes of psychedelics on the growth of both dendrites and dendritic spines, and Moda-Sava et al.16 were able to show that the ketamine-induced restoration of dendritic spines in neurons of the prefrontal cortex is essential for its long-term effects in mice. As the psychedelic renaissance evolves and interest and funding increase19, some of the key questions outlined above will hopefully be addressed using more powerful neuroimaging techniques and analysis algorithms. The research community will also be able to produce important insights into the molecular mechanisms of psychedelics using a wide range of new technologies. By combining genetic engineering and neuroscience, they will be able to better understand how psychedelics impact neuronal morphology and synaptic connectivity, and therefore overall brain activity. Such research will surely also lead to insights on how these substances bring about their therapeutic effects. This will benefit not only the quest for new treatments for psychiatric disorders, but also the understanding of the neurobiological basis of consciousness and self.
If you share our vision and want to support psychedelic research and education, we are grateful for any amount you can give.
1. GBD 2017 Disease and Injury Incidence and Prevalence (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159):1789-1858
2. Frazer, A., & Benmansour, S. (2002). Delayed pharmacological effects of antidepressants. Molecular Psychiatry, 7, s23-s28.
3. Arroll, B., Macgillivray, S., Ogston, S., Reid I., Sullivan, F., Williams, B., Crombie I. (2005). Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: A meta-analysis. Annals of Family Medicine, 3(5): 449 – 456.
4. Joshi A. (2018). Selective serotonin re-uptake inhibitors: an overview. Psychiatria Danubia, 30(7):605-609.
5. McEwen, B. S., Eiland, L., Hunter, R. G., Miller, M. M. (2012). Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology, 62(1):3-12.
6. Radley, J. J., Morrison, J. H. (2005). Repeated stress and structural plasticity in the brain. Ageing Research reviews, 4(2):271-287.
7. Patel, D., Anilkumar, S., Chattarji, S., Buwalda, B. (2018). Repeated social stress leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Behavioural Brain Research, 347:314-324.
8. Christoffel, D. J., Golden, S. A., Russo, S. J. (2011). Structural and synaptic plasticity in stress-related disorders. Rev. Neurosci. 22:535 – 549.
9. Lau, T., Bigio, B., Zelli D., McEwen, B. S., Nasca, C. (2017). Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant. Molecular Psychiatry, 22: 227 -234.
10. Mithoefer, M. C., Wagner, M. T., Mithoefer, A. T., Jerome, L., Doblin, R. (2011) The safety and efficacy of MDMA-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. Journal of Psychopharmacology, 25(4):439 – 452.
11. Carhart-Harris, R. L., Bolstridge, M., Rucker, J., Day, C. M., Erritzoe, D., Kaelen, M., Bloomfield, M., Rickard, J. A., Forbes, B., Feilding, A., Taylor, D., Pilling, S., Curran, V. H., Nutt, D. J. (2016). Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry, 3(7):619 – 627.
12. Ly, C., Greb, A. C., Cameron, L. P., Wong J. M., Barragan, E. V., Wilson, P. C., Burbach, K. F., Soltanzadeh Zarandi, S., Sood, A., Paddy, M. R., Duim, W. C., Dennis M. Y., McAllister, A. K., Ori-McKenney, K. M., Gray, J. A., Olson, D. E. (2018). Psychedelics promote structural and functional neural plasticity. Cell Reports, 23(11):3170 – 3182.
13. Lu, B., Nagappan, G., Lu, Y. (2014). BDNF and synaptic plasticity, cognitive function, and dysfunction. Neurotrophic Factors book chapter, Handbook of experimental pharmacology, 220: 223 – 250.
14. Phillips, C. (2017). Brain-derived neurotrophic factor, depression, and physical activity: Making the neuroplastic connection. Neural Plasticity, 2017:7260130.
15. Martinowich, K., Manji, H., Lu, B. (2007). New insights into BDNF function in depression and anxiety. Nature Neuroscience, 10: 1089 – 1093.
16. Moda-Sava, R. N., Murdock, M. H., Parek, P. K., Fetcho, R. N., Huang, B. S., Huynh, T. N., Witztum, J., Shaver, D. C., Rosenthal D. L., Alway, E. J., Lopez, K., Meng, Y., Nellissen, L., Grosenick, L., Milner, T. A., Deisseroth, K., Bito, H., Kasai, H., Liston, C. (2019). Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science, 364(6436). pii:eaat8078.
17. Beyeler, A. (2019). Do antidepressants restore last synapses? Science, 364(6436):129 – 130.
18. Carhart-Harris, R. L., Muthukumaraswamy, S., Roseman, L., Kaelen, M., Droog, W., Murphy, K., Tagliazucchi, E., Schenberg, E. E., Nest, T., Orban, C., Leech, R., Williams L. T., Williams, T. M., Bolstridge, M., Sessa, B., McGonigle, J., Sereno, M. I., Nichols, D., Hellyer, P. J., Hobden, P., Evans J., Singh K. D., Wise, R. G., Curran, H. V., Feilding A., Nutt, D. J. (2016). Neural correlates of the LSD experience revealed by multimodal neuroimaging. PNAS, 113(17):4853 – 4858.
19. Gründer, G., 2020. German Government Funds Psilocybin Study for Depression. [Blog] Available at: https://www.mind-and-brain.institute/psychedelics/german-government-funds-psilocybin-study-for-depression/ [Accessed 17 June 2020].